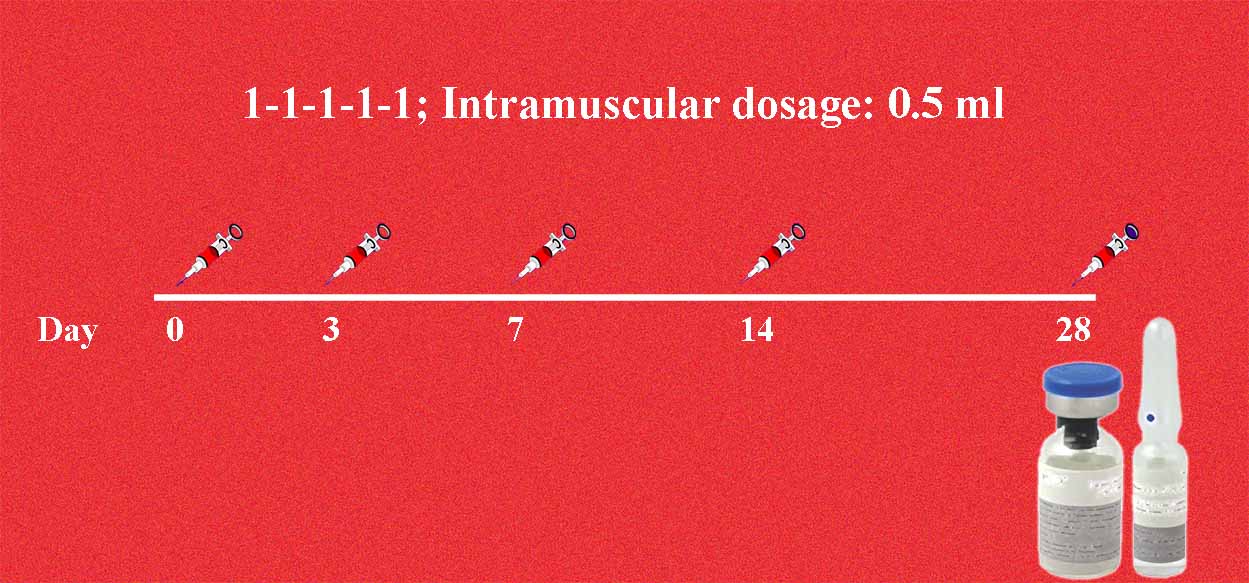
A New Chromatographically Purified Rabies Vaccine
SPEEDA®
For Human Use (Vero Cell)
(CPRV)
SPEEDA is a highly purified next-generation rabies vaccine, refined through chromatography and produced from the PV-2016 rabies virus strain (a Pasteur strain supplied by the U.S. CDC and recommended by the WHO), based on the continuous Vero cell culture principle.
SPEEDA has been available on the market since 2005, contributing to the supply of next-generation vaccines to provide rabies immunity for people in Vietnam and globally. Most recently, this vaccine has been approved for intradermal administration using the TRC-ID regimen in Thailand.
Note: Any animal, including dogs, cats, bats, rats, etc., that bites must be vaccinated!
Using the new-generation Vero cell rabies vaccine SPEEDA® is the best way to protect yourself against rabies.